The FDA has announced a Class I recall of certain models of Medtronic implantable defibrillators and cardiac resynchronization therapy defibrillators. The statement from the FDA can be found here.
The recall impacts 348,616 devices implanted between October 13, 2017 to June 9, 2023 with the following models being affected:
- Cobalt XT, Cobalt, Crome ICDs and CRT-Ds
- Claria MRI, Amplia MRI, Compia MRI, Viva, Brava CRT-Ds
- Visia AF, Visia AF MRI, Evera, Evera MRI, Primo MRI, Mirro MRI ICDs
These devices fail to deliver therapy due to inappropriate activation of the Short Circuit Protection feature. The issue is more likely to occur for devices with a glassed feedthrough that are configured to deliver therapy in the AX>B delivered pathway.
A reduced-energy shock, or no shock at all, may fail to correct a life-threatening arrhythmia, which can lead to cardiac arrest, other serious injury, or death.
There have been 28 incidents, 22 injuries, and no deaths for this issue.
A software update delivered to your device will address the issue. This requires a visit to your health care practitioner for a quick update. I had mine updated in a quick 10 minute visit to my ICD clinic.
To look up your device by product name, model or serial number to see whether it is impacted, click here.
NOTE: You can find the name, model and serial number of your device on the plastic identification card provided to you at the time of your implant.
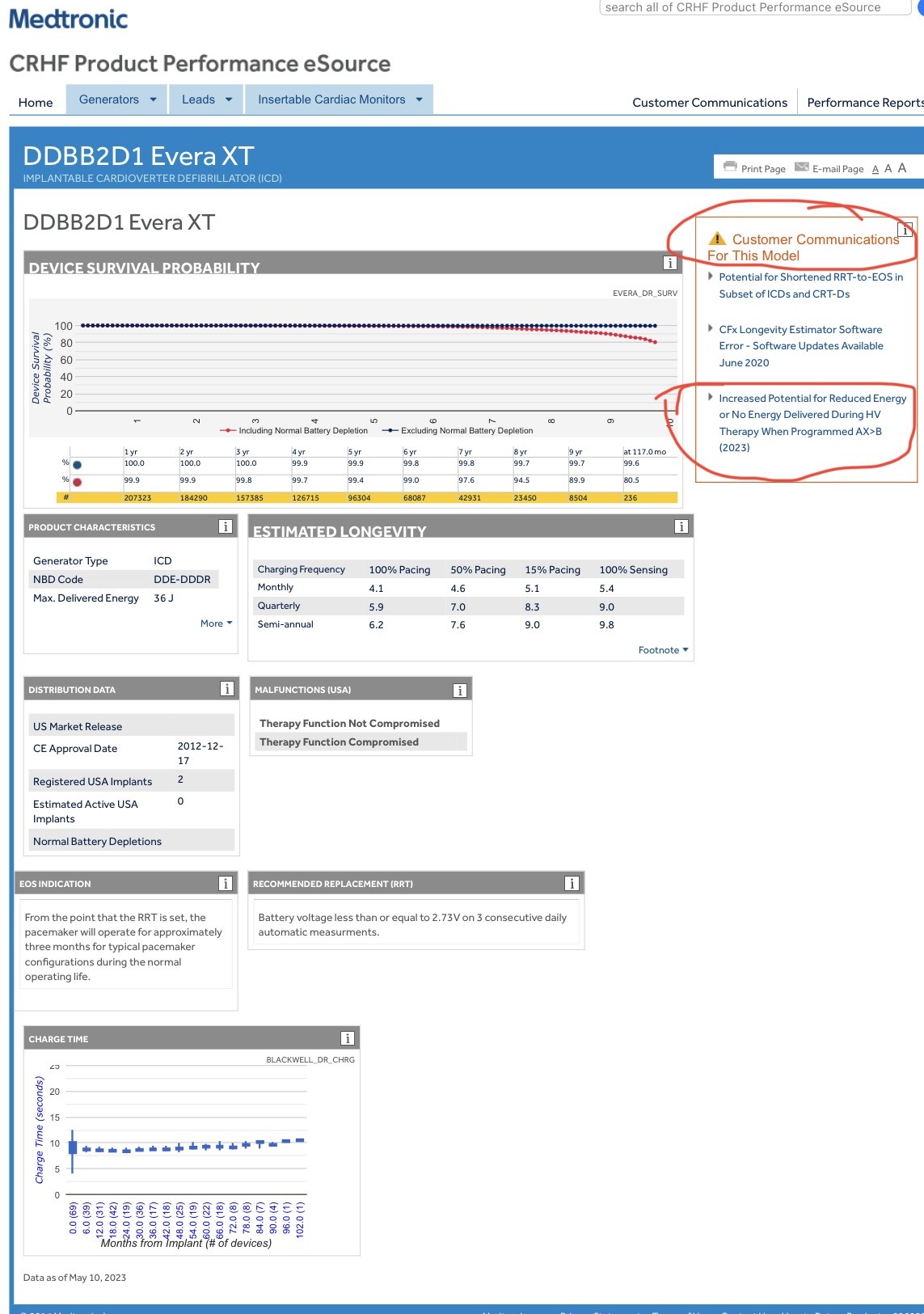
The box in the upper right corner labeled “Customer Communications For This Model” will tell you if there are any advisories for your device. If this particular recall affects your device, you will see the same advisory which is circled in red in the photo below.
Patients are advised to continue routine follow ups and use the Care Link Monitoring System.
And, as always, you can call Medtronic Patient Services with any questions at: (800) 551-5544
(M – F, 8am – 5pm Central).





